Search Posts
Recent Posts
- In the news: Quick recap of the week’s news… 6.14.25 June 14, 2025
- Drowning in corruption in Bonnet Shores: Hundreds sign petition. No action by lawmakers. June 14, 2025
- Rhode Island Weather for June 14, 2025 – Jack Donnelly June 14, 2025
- Burn with Kearns: Train smarter, not harder. Multi-use fitness tools – Kevin Kearns June 14, 2025
- Out & About in RI: Miriam Hospital Gala raises over $890,000 June 14, 2025
Categories
Subscribe!
Thanks for subscribing! Please check your email for further instructions.
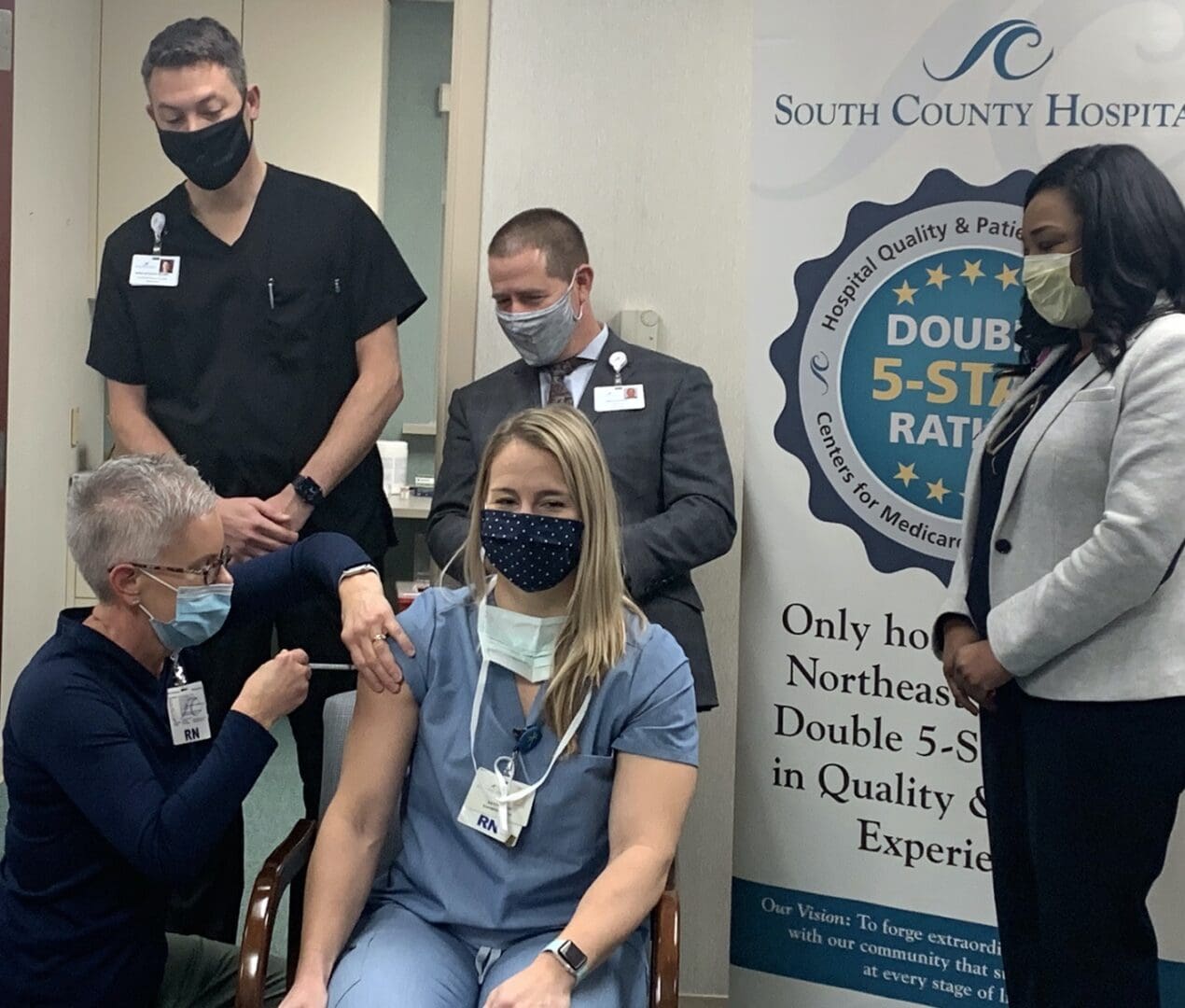
Vaccines begin for staff at South County Health
Photo: Members of South County Health’s Executive Leadership celebrate the first COVID-19 vaccine administered at South County Health.
250 staff signed up for the first of eight clinics to receive the Pfizer vaccine
On Friday, December 18, 2020, Kevin Hurley, a registered nurse who works in the emergency department at South County Hospital become the first healthcare worker at South County Health to receive the recently approved Pfizer vaccine for COVID-19.
Over the course of eight scheduled vaccine clinics, Hurley is to be followed by approximately 1,060 of her colleagues who signed up to receive the first of two doses of the Pfizer vaccine.
The vaccine was delivered to South County Hospital on Thursday, December 17, the vials packed in dry ice to keep the drug at the required -94 degrees F.
The shipment contained enough supply to inoculate every staff member with the first dose, said Drew Ross, PharmD, MBA, Pharmacy Manager at South County Health.
Shortly before the first staff members started to arrive for the 10 a.m. start of the clinic, pharmacists began mixing the vials with a saline diluent to ensure the proper dosage.
“Based on the information we received from the Centers for Disease Control and the RI Department of Health, we are confident that the Pfizer and Moderna vaccines will be pivotal in controlling the COVID-19 virus,” said Aaron Hattaway, MD, Chief Medical Officer.
“We have been assured that no steps in the evaluation of safety or efficacy were skipped in the development of this vaccine. Due to the immense public need, significant financial and human resources were made available to conduct these trials as quickly as possible,” Hattaway said.
Usual procedural delays between steps were abbreviated by executive order resulting in less waiting between steps, but no steps skipped.
According to the research leading up to the distribution of the COVID vaccine, there is a chance that side effects such as fever, aches, sore arm, body malaise may be experienced, particularly after receiving the second of two doses.
With the eight vaccine clinics, staff in each department are scheduled on different days to ensure that no department is left understaffed should someone have an adverse reaction or side-effect to the vaccine. At the time staff receive the first dose of the vaccine, an appointment will be scheduled for them to receive the second dose 21 days later.
South County Health president/CEO Aaron Robinson reflected on the importance of this step in protecting healthcare workers and the general public.
“Our team is excited to embark on this momentous administration of the COVID-19 vaccine. This will be critical to protect our frontline staff who have worked tirelessly to serve our community. After review of the Pfizer vaccine, we believe the vaccine to be safe, highly effective, and a promising turning point in the battle against COVID-19.,” Robinson said.
COVID-19 Vaccine Information
On Monday, December 14, 2020, South County Health held a staff virtual Town Hall meeting via Zoom, led by Dr. Philip Chan of the RI Department of Health and South County Health’s Vaccine Task Force – Andrew Ross, Pharm.D.; Drew Murphy; Lee Ann Quinn, RN, Infection Prevention Director; Stephanie Parente, RN, Clinical Leader, Infection Prevention & Employee Health; Anitra Galmore, Chief Nursing Officer; Dr. Aaron Hattaway, Chief Medical Officer; and Nathan Kinsella, RN.
The purpose of the Vaccine Task Force was to prepare for the distribution of the vaccine at South County Health with planning and logistics. That team began to meet in November, to ensure resources and processes were in place even before the vaccine received FDA approval.
Prior to the Town Hall session, staff were asked to submit questions regarding the COVID vaccine to be answered by the panel of experts.
The questions and responses appear below.
Is it safe to give COVID vaccine (Pfizer, Moderna) to pregnant or women planning to become pregnant?
The Emergency Use Authorization (EUA) allows pregnant women to be offered the vaccine. There is very little data around the use of this vaccine in the pregnant population. The risks and benefits should be weighed by the employee, in conversation with their primary care physician or obstetrician.
I heard the vaccine DNA can attack spiked proteins similar to proteins that are found in the placenta and can be damaging.
As above.
Is there any concern or knowledge regarding receiving the vaccine and becoming pregnant sometime soon after receiving the vaccine?
As above.
Has research been done around the vaccine and how it affects those with autoimmune conditions?
Patients with autoimmune diseases, and immune deficiencies including Hepatitis B, Hepatitis C, and HIV/AIDS were included in the trials. No significant differences were observed in safety or efficacy in these groups compared to the general population. Based on data from other vaccines, it is possible that immunocompromised persons may have a diminished response to the vaccine (ie. It may be less effective in this group).
Are we still going to have to quarantine and follow current COVID prevention practices after an exposure once we are vaccinated?
This may change, but at this time the usual quarantine period will still apply and COVID prevention must still be practiced (masking, hand hygiene, social distancing, etc.).
Is there a mechanism to confirm the vaccine has effectively produced antibodies, particularly in staff with underlying immune issues? IgM &/or IgG antibody testing
There is no recommendation for this sort of monitoring at this time.
Allergies/Interactions
Vaccines administered in the UK found there to be issues will allergic reactions in some patients. Can you elaborate more on that?
In the course of administering tens of thousands of doses to a population consisting mostly of healthcare workers, two vaccine recipients experienced anaphylactic reactions. This can occur with any vaccine and appears to be a rare adverse event.
I have been hearing that people with allergies should not get the vaccine. Do you have any info regarding this vaccine? I get the flu shot every year but have a lot of other allergies.
The only group of patients we are recommending additional monitoring or consideration of postponing vaccination in is patients who have had previous anaphylactic reactions to another vaccine or injectable medication.
Once the injection is given is there a period of waiting to see if a reaction develops?
In most patients, we will monitor you for 15 minutes for reaction. In the group of patients above (previous anaphylactic reactions to another vaccine or injectable medication) we will monitor you for 30 minutes.
If a person has taken the first of two shingles vaccinations and is due for the second shot in the time between the two COVID vaccine doses, is it still okay to receive the first shot of the COVID vaccine too?
Each COVID vaccine should be spaced at least 14 days apart from any other vaccines.
Is this vaccine safe for those who have egg allergies?
The COVID vaccine does not contain egg.
Can I get the vaccine if I take Humira?
Please contact the provider who prescribes Humira (or any other medication of concern) and discuss the risks and benefits.
I had an allergy to penicillin and erythromycin when I was in my 20s. I’m in my 50s and I haven’t had any reactions recently but just wondering if that is an issue.
The vaccine does not contain penicillin or erythromycin. If you have ever experienced an anaphylactic reaction to an injectable medication, we will monitor you for 30 minutes (rather than the usual 15) for reaction following your injection.
What if you have asthma? Will you still be able to get the vaccine?
Yes, patients with lung disease were included in the clinical trials and saw no significant difference in efficacy or safety.
Reactions/Side Effects
What are the expected side effects to the vaccine that are different than an allergic reaction?
In the clinical trials there were the following: 84% pain at injection site, 63% fatigue, 55% headache, 38% malaise, 32% chills, and 14% fever.
If I get the vaccine, and then experience side effects such as fever, aches, cough, should I stay home from work until my symptoms resolve?
There is a chance you may experience side effects such as fever, aches, sore arm, body malaise. It is advisable to schedule your vaccination 24-48 hours prior to a day off in case you do not feel well enough to work. Because these side effects also are similar with COVID illness, you may want to have a COVID test during that day or next day to ensure you do not have COVID at the same time as getting vaccinated. The vaccine does not give you COVID illness and will not cause you to test positive, it does not work that way.
COVID+ /PUI persons
If you previously had COVID-19 and fully recovered, particularly within the last 3 months, should you still receive the vaccine?
Yes. However, you should not receive the COVID-19 vaccine within 14 days of active infection (you must wait until your isolation period ends).
Can we have a COVID antibodies test before taking the vaccine?
Getting antibody tested to suggest you have antibodies to make a decision on whether you should be vaccinated is not a good indicator for that decision because natural immunity from COVID-19 after infection may wane after 90 days.
Future planning
Will the COVID vaccine be offered annually?
At this time, there is (limited) evidence to suggest that the vaccine may offer multiple years of protection. Whether an additional “booster” dose will be required is still being evaluated.
What steps did they/are they skipping in the creation and testing process for the vaccine, that are typically required important steps with other vaccine and med approvals? Why was this one able to be processed and approved so quickly in comparison to others in the past?
No steps in the evaluation of safety or efficacy were skipped in the development of this vaccine. Due to the immense public need, significant financial and human resources were made available to conduct these trials as quickly as possible. Usual procedural delays between steps were abbreviated by executive order (less waiting between steps, but no steps skipped).
Long term side effects – particularly of the one that changes the RNA?
Long-term side effects, from a theoretical medical perspective, are unlikely since two very low doses of a highly unstable (leaves your body quickly) molecule are administered. Trials began in July, and as such, we only have data spanning (at most) 6 months, so there is no long-term data, but the short-term safety data is encouraging.
How is efficacy determined? I hear that Pfizer’s vaccine has 95% efficacy – how do they know that?
Assuming a COVID exposure 7 days or more after your second vaccine dose, you are 95% less likely to get sick that if you did not have the vaccine.
When will the COVID vaccine be available to the general public?
Prioritization and distribution of the vaccines will be scheduled by the Centers for Disease Control and state’s departments of health. As of Dec. 14, 2020, no schedule has been released beyond the vaccine made available to healthcare workers.
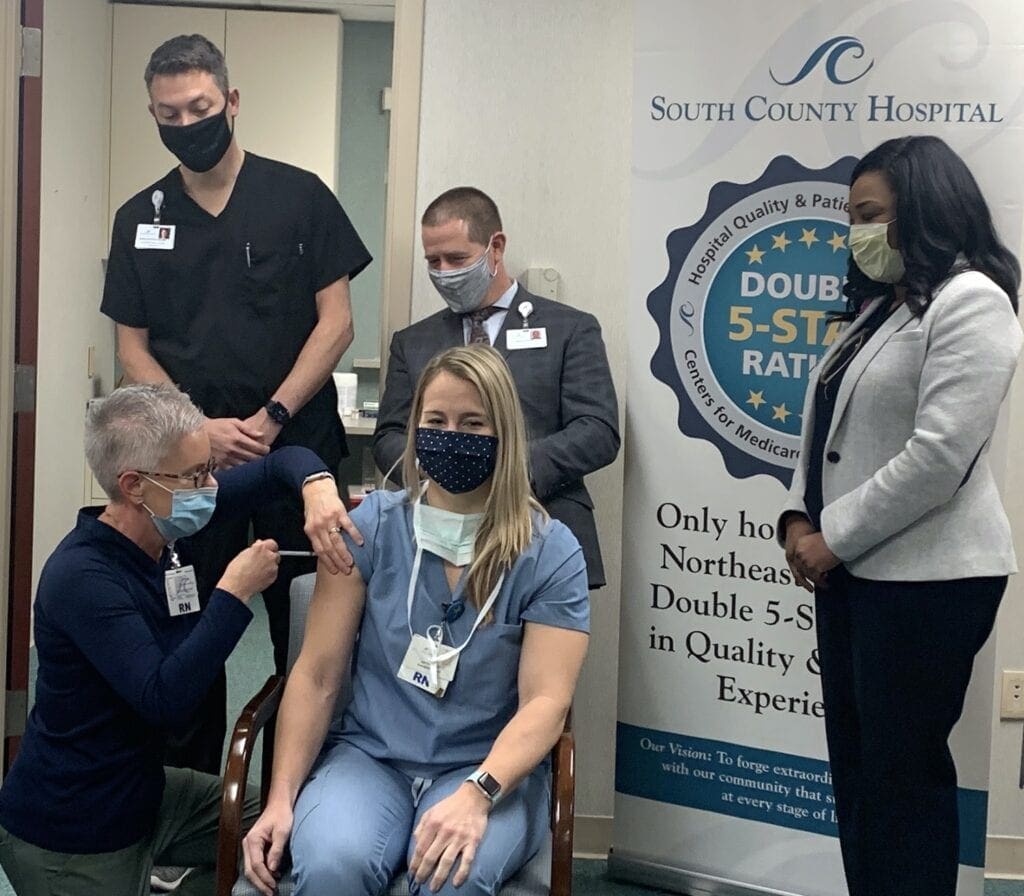
Kevin Hurley, RN, who works in South County Hospital’s Emergency Department is the first South County Health staff to receive the COVID-19 vaccine. Administering the vaccination is Mary Ellen Casey, RN, with onlookers (left to right) Dr. Arron Hattaway, MD, Chief Medical Officer; Aaron Robinson, South County Health president/CEO; Anitra Galmore, RN, BSN, NEA-BC, Vice President, Chief Nursing Officer/Chief Operations Officer.
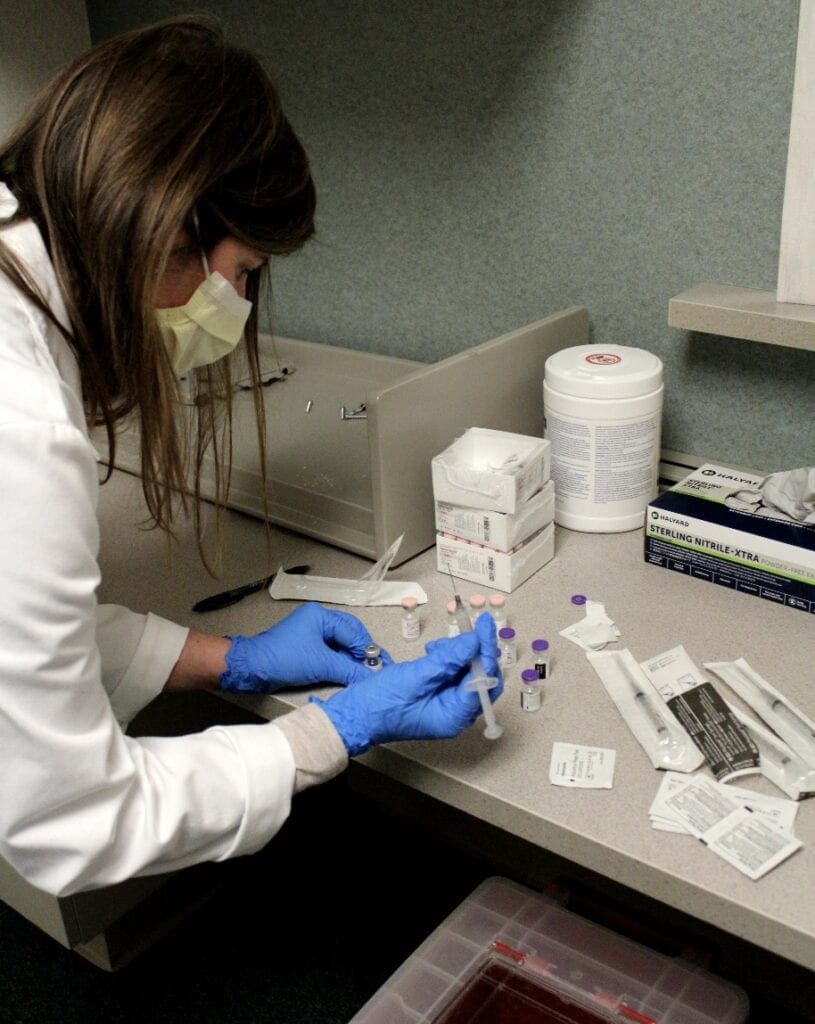
Marissa Palm, PharmD mixes the COVID-19 vaccine with a saline solution to prepare the correct dosage.

Jackie Constantino, BS. Pharm, MBA, BCSC, mixes the COVID-19 vaccine with a saline solution to prepare the correct dosage.
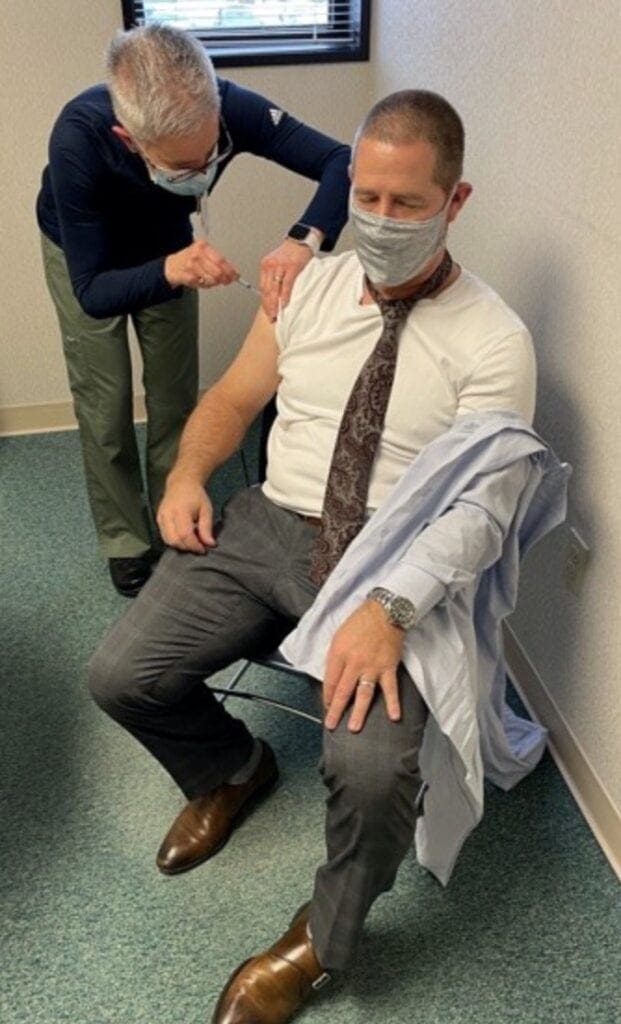
South County Health president/CEO Aaron Robinson demonstrated unity among healthcare workers in the battle against COVID-19, being among the staff who received the vaccine.
