Search Posts
Recent Posts
- Rhode Island Weather for June 15, 2025 – Jack Donnelly June 15, 2025
- To honor Pawtucket Mayor Henry Kinch: A tribute to leadership and legacy June 15, 2025
- Ask Chef Walter: Summer Feast for the Palate – Chef Walter Potenza June 15, 2025
- A Greener View: Floppy Perennials, Holey Vegetables and Wet Soil Gardening – Jeff Rugg June 15, 2025
- Gimme’ Shelter: Old Bay at the Providence Animal Control Center June 15, 2025
Categories
Subscribe!
Thanks for subscribing! Please check your email for further instructions.
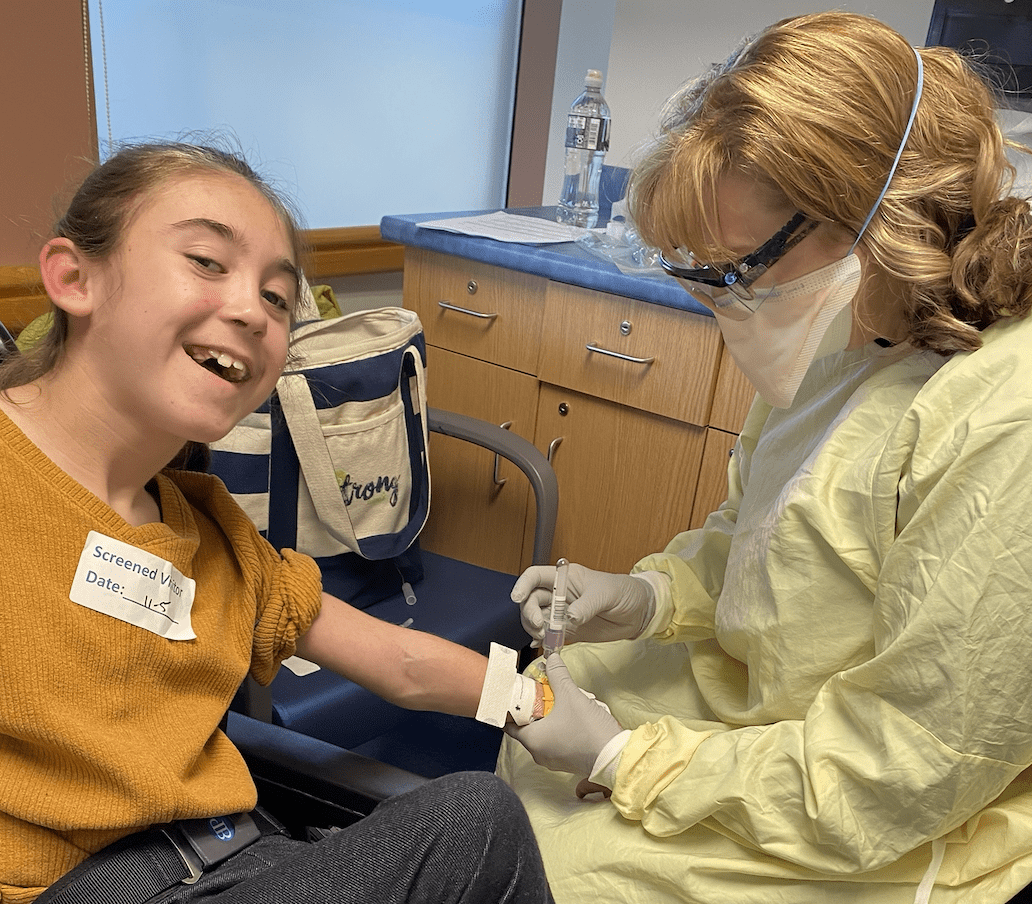
Hasbro Children’s Hospital first in region to treat children (0-11) with monoclonal antibodies
Photo: Lifespan
Hasbro Children’s Hospital held a virtual press conference to address COVID-19 treatment in children. Noting that they are the first hospital in the region to get emergency use authorization monoclonal antibody treatments, treatments are currently underway for children of all ages.
Last week the FDA expanded emergency use authorization of two monoclonal antibodies to treat the youngest of pediatric patients (0-11 years) with COVID-19 and at risk of severe COVID.
Hasbro patient Abigail MacCurtain, a young, high-risk patient, at her follow-up apppointment after receiving her monoclonal antibody treatment, is seen, above.

Emergency Use Authorization Treatments
Monoclonal antibody treatment is now offered under emergency use authorization for children ages 0-18 years.
The treatment is available under one of the following circumstances:
- The patient has a positive test for COVID-19 and onset of symptoms within 10 days, or
- The patient has a confirmed exposure to COVID-19
Eligibility Requirements
Treatment is for outpatients only, those with mild to moderate symptoms not requiring oxygen or hospitalization. Infusions are performed at the Tomorrow Fund Clinic at Hasbro Children’s Hospital.
High-Risk Consideration
To be considered high risk for developing severe COVID-19, a patient must have one or more of the following risk factors:
- Age less than 12 months
- A BMI greater than or equal to the 85th percentile for their age and gender based on CDC growth charts
- Immunosuppressive disease or immunosuppressive treatment
- Cardiovascular disease, including congenital heart disease and hypertension
- Chronic lung diseases (chronic obstructive pulmonary disease, asthma, interstitial lung disease, cystic fibrosis, pulmonary hypertension)
- Sickle cell disease or thalassemia
- Neurodevelopmental disorders like cerebral palsy or other conditions that confer medical complexity, like metabolic syndromes and severe congenital anomalies
- A medical-related technological dependence unrelated to COVID (tracheostomy, gastrostomy, positive pressure ventilation)
- Diabetes (types 1 and 2)
- Pregnancy
- Chronic kidney disease
- Chronic liver disease
Refer a Patient
Physicians with a patient who may qualify for this treatment, please complete the MAB Intake Form (PDF) and fax it to 401-444-5650.
More Information
For more information, contact your child’s primary care pediatrician or contact the division of pediatric infectious diseases at 401-444-8360.
