Search Posts
Recent Posts
- Rhode Island Weather for June 1, 2025 – Jack Donnelly June 1, 2025
- To Do in RI: 26th Annual Rose Show of the Rhode Island Rose Society June 1, 2025
- Victory is ours: Victory gardens are blossoming again – Chuck Norris June 1, 2025
- Ask Chef Walter: The problem with “The Best” – Chef Walter Potenza June 1, 2025
- Gimme’ Shelter: Kava is waiting at the Providence Animal Control Center June 1, 2025
Categories
Subscribe!
Thanks for subscribing! Please check your email for further instructions.
FDA Says “Go!” to Moderna COVID19 Vaccine
Friday night, the U.S. Food and Drug Administration issued an emergency use authorization for the second vaccine for the prevention of COVID-19. The emergency use authorization allows the Moderna COVID-19 Vaccine to be distributed in the U.S. for use in individuals 18 years of age and older.
From the FDA:
“With the availability of two vaccines now for the prevention of COVID-19, the FDA has taken another crucial step in the fight against this global pandemic that is causing vast numbers of hospitalizations and deaths in the United States each day,” said FDA Commissioner Stephen M. Hahn, M.D. “Through the FDA’s open and transparent scientific review process, two COVID-19 vaccines have been authorized in an expedited timeframe while adhering to the rigorous standards for safety, effectiveness, and manufacturing quality needed to support emergency use authorization that the American people have come to expect from the FDA. These standards and our review process, which are the same we have used in reviewing the first COVID-19 vaccine and intend to use for any other COVID-19 vaccines, included input from independent scientific and public health experts as well as a thorough analysis of the data by the agency’s career staff.”
The FDA has determined that the Moderna COVID-19 Vaccine has met the statutory criteria for issuance of an EUA. The totality of the available data provides clear evidence that the Moderna COVID-19 Vaccine may be effective in preventing COVID-19. The data also show that the known and potential benefits outweigh the known and potential risks—supporting the company’s request for the vaccine’s use in people 18 years of age and older. In making this determination, the FDA can assure the public and medical community that it has conducted a thorough evaluation of the available safety, effectiveness, and manufacturing quality information.
The Moderna COVID-19 Vaccine contains messenger RNA that instructs cells in the body to make the virus’s distinctive “spike” protein. After a person receives this vaccine, their body produces copies of the spike protein, which does not cause disease, but triggers the immune system to learn to react defensively, producing an immune response.
“Guided by science and data, the agency’s career staff determined that the vaccine’s known and potential benefits clearly outweigh its known and potential risks, and although not an FDA approval, the FDA’s expectations described in our June and October guidance documents have been met,” said Peter Marks, M.D., Ph.D., Director of the FDA’s Center for Biologics Evaluation and Research. “Today’s authorization demonstrates our steadfast commitment to the health of the American people, with the assurance that our scientific standards and the integrity of our review process have been maintained. This achievement is yet another testament to the dedication of FDA’s career scientists and physicians, who have been working urgently to conduct comprehensive and rigorous evaluations of the data submitted for vaccines to prevent COVID-19.”
From Moderna:
U.S. FDA authorizes mRNA vaccine against COVID-19 for emergency use, with a total of 200 million doses ordered by the U.S government to date; U.S. government retains option to purchase up to an additional 300 million doses – approximately 20 million doses will be delivered by the end of December 2020
Delivery to the U.S. Government will begin immediately. Moderna will continue to gather additional data and plans to file a Biologics License Application with the FDA requesting full licensure in 2021.
“I want to thank the thousands of participants in our clinical trials and the staff at our clinical trial sites who have been on the front lines of the fight against the virus. I want to thank the NIH and NIAID for their scientific leadership and our partners at BARDA and Operation Warp Speed who have been instrumental to accelerating our progress to this point. I also want to thank the Moderna team, our suppliers and our partners for their tireless work across research, development and manufacturing of our vaccine,” said Stéphane Bancel, Chief Executive Officer of Moderna. “I am proud of what the Moderna team has achieved in collaboration with our partners. We were able to create and manufacture the Moderna COVID-19 Vaccine in 11 months from sequence to authorization, while advancing clinical development with a Phase 1, Phase 2 and pivotal Phase 3 study of 30,000 participants. It has been a 10-year scientific, entrepreneurial and medical journey and I am thankful to all those who have helped us get here today. We remain focused on scaling up manufacturing to help us protect as many people as we can from this terrible disease.”
The primary efficacy analysis conducted on 196 cases indicated a vaccine efficacy rate of 94.1%.
Under Operation Warp Speed, the Department of Defense in partnership with the Department of Health and Human Services and the U.S. Centers for Disease Control and Prevention, will manage allocation and distribution of the vaccine in the United States. Allocation and distribution will be prioritized according to populations identified by the CDC’s Advisory Committee on Immunization Practices.
Approximately 20 million doses will be delivered to the U.S. government by the end of December 2020. The Company expects to have between 100 million and 125 million doses available globally in the first quarter of 2021, with 85-100 million of those available in the U.S.
Moderna would also like to underscore the importance of the CARES Act — and thank its bipartisan Congressional champions — specifically with respect to its significant commitment in funding to enable BARDA and Operation Warp Speed to simultaneously fund development and manufacturing ahead of approval.
Packing up:
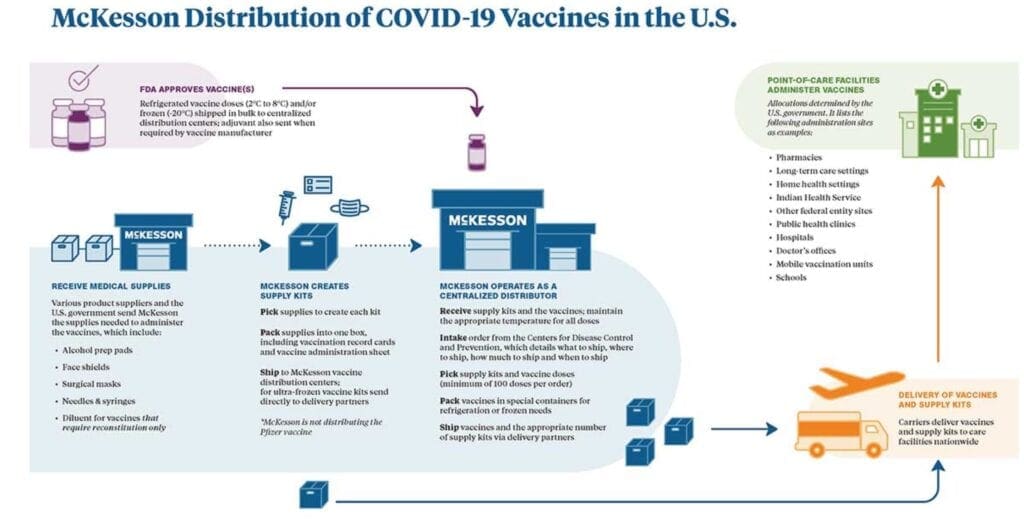
Medical supply company McKesson will obtain the doses from Moderna for packaging and distribution. A global leader in the healthcare industry, McKesson Corporation says “we will expand its existing partnership with the Centers for Disease Control (CDC) to support the U.S. government’s Operation Warp Speed (OWS) team as a centralized distributor of future COVID-19 vaccines and ancillary supplies needed to administer vaccinations. Vaccines and related supplies will be delivered to point-of-care sites across the country at the U.S. government’s direction.”
US Placed a First & Second Order
On Dec. 11th, Moderna announced that the U.S. government has exercised its option to purchase an additional 100 million doses of mRNA-1273, Moderna’s COVID-19 vaccine candidate, bringing its confirmed order commitment to 200 million doses.
Of the first 100 million doses purchased by the U.S. government, approximately 20 million doses will be delivered by the end of December 2020 and the balance will be delivered in the first quarter of 2021. Today’s new order of 100 million doses will be delivered in the second quarter of 2021. These deliveries are subject, in each case, to receipt of an Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the vaccine.
European Commission Exercises Option for Additional 80 Million Doses of Moderna’s COVID-19 Vaccine Candidate
The European Commission has exercised its option to purchase an additional 80 million doses of mRNA-1273, Moderna’s COVID-19 vaccine candidate, bringing its confirmed order commitment to 160 million doses.
The first deliveries of mRNA-1273 to European countries from Moderna’s dedicated European supply chain are expected to commence early in 2021 following regulatory approval by the EMA.
“We appreciate the confidence in Moderna and mRNA-1273, our COVID-19 vaccine candidate, demonstrated by today’s increased supply agreement with the European Commission” said Stéphane Bancel, Chief Executive Officer of Moderna. “As we shift our focus now to prepare for the delivery of our vaccine candidate, pending a positive opinion from the EMA and other regulators, we remain committed to working with governments and partners globally to address this pandemic.”
Moderna’s COVID-19 Vaccine Supply Agreements
Moderna has confirmed the following supply agreements of committed orders totaling more than 470 million doses:
- United States: 200 million doses with option for an additional 300 million doses
- European Union: 160 million doses
- Japan: 50 million doses
- Canada: 40 million doses with option for an additional 16 million doses
- Switzerland: 7.5 million doses
- United Kingdom: 7 million doses
- Israel: 6 million doses
- Qatar
- Singapore
- Other countries, which have placed orders and have not been disclosed.
