Search Posts
Recent Posts
- Homeless in RI: “We’re Terrified…” says Coalition. Lacks Water, Supplies for Heat Wave. RIEMA Cooling Center list. June 20, 2025
- Providence Delivers Summer Fun: Food, Water Play & Activities June 20, 2025
- Rhode Island Weather for June 20, 2025 – Jack Donnelly June 20, 2025
- Outdoors in RI: Time to Seastreak – Vets Access – Invasive Alerts – Clays 4 Charity at The Preserve – 2A TODAY June 20, 2025
- GriefSPEAK: Kill Switch – Mari Nardolillo Dias June 20, 2025
Categories
Subscribe!
Thanks for subscribing! Please check your email for further instructions.

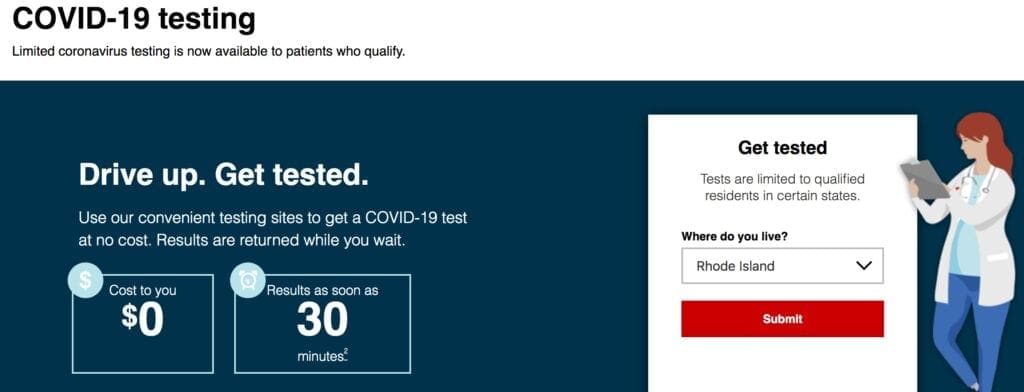
CVS Health opening rapid COVID-19 drive-through testing in Rhode Island
Use of the Abbott ID NOW COVID-19 test will provide on-the-spot test results – between 2 and 30 minutes.
To help support local communities and the overall health care system in addressing the COVID-19 pandemic, CVS Health joined forces with federal and state officials to announce the opening of rapid COVID-19 drive-through testing sites in Rhode Island and in Georgia. The test sites will bolster state efforts to manage the spread of the virus and provide on-the-spot test results.
CVS Health will utilize licensed health care providers from MinuteClinic, the company’s retail medical clinic, to oversee the testing, which is currently available at no-cost to patients. The company is applying the significant learnings gathered from its COVID-19 testing site opened in Shrewsbury, Massachusetts on March 19, to help maximize the efficiency and safety at these new sites. For example, testing at these new sites will be held in large parking lots that are easily accessible and able to accommodate multiple lanes of cars at one time and will require eligible individuals to pre-register online. COVID-19 testing will not take place at CVS Pharmacy or MinuteClinic locations.
“Our MinuteClinic providers join countless other heroic health care professionals across the country and around the world in forming the first line of defense against this devastating virus,” said Troyen Brennan, MD, MPH, Chief Medical Officer and Executive Vice President, CVS Health. “Thanks to our partnerships with state officials and the utilization of advanced technology, our providers will be able to test large numbers of people in these states and make real-time decisions about treatment and appropriate next steps.”
The rapid testing will be conducted using the new Abbott ID NOW COVID-19 test, which recently received emergency use authorization from the U.S. Food and Drug Administration for the fastest available molecular point-of-care test for the detection of COVID-19. Positive results can be delivered in as little as five minutes and negative results in as little as 13 minutes.
Approximately 2,000 tests a day are planned at this site.
Rapid COVID-19 testing will be available to eligible individuals who meet criteria established by the Centers for Disease Control and Prevention, in addition to state residency and age guidelines. Patients will need to pre-register in advance online at CVS.com in order to schedule a same-day time slot for testing.
The test site, in RI, will be located at Twin River Casino (100 Twin River Road, in Lincoln. Please visit CVS.com.
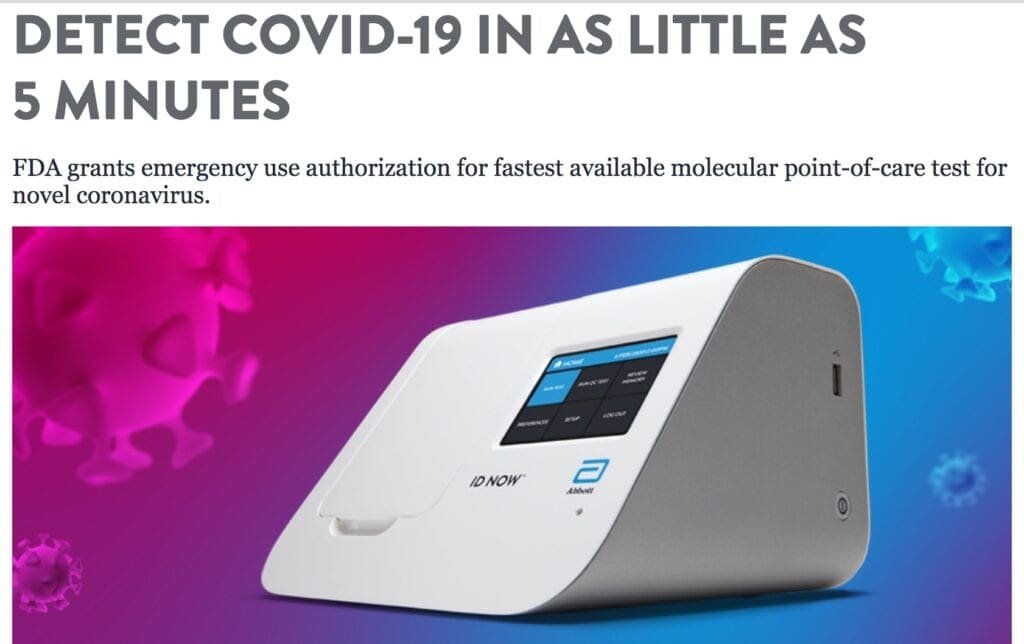
Abbott ID NOW COVID-19
Abbott has received emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the fastest available molecular point-of-care test for the detection of novel coronavirus (COVID-19), delivering positive results in as little as five minutes and negative results in 13 minutes.
What makes this test so different is where it can be used: outside the four walls of a traditional hospital such as in the physicians’ office or urgent care clinics.
The new Abbott ID NOW COVID-19 test runs on Abbott’s ID NOWTM platform—a lightweight box (6.6 pounds and the size of a small toaster) that can sit in a variety of locations.
Because of its small size, it can be used in more non-traditional places where people can have their results in a matter of minutes, bringing an alternate testing technology to combat the novel coronavirus.
We’re ramping up production to deliver 50,000 ID NOW COVID-19 tests per day, beginning next week, to the U.S. healthcare system.
This comes on the heels of our announcement last week of the availability of the Abbott RealTime SARS-CoV-2 EUA test under FDA EUA, which runs on m2000 RealTime molecular system for centralized lab environments. Combined with ID NOW, Abbott expects to produce about 5 million tests in April.
Testing remains a crucial step in controlling the novel COVID-19 pandemic. Continuing to supply healthcare providers with new technologies to help curb the spread of infection is a top priority for public health officials and healthcare providers.
Taking molecular testing to the front lines
Molecular point-of-care testing for COVID-19 offers healthcare workers rapid results in more settings where people show up for care. Molecular testing technologies help detect the presence of a virus by identifying a small section of the virus’ genome, then amplifying that portion until there’s enough for detection. This process can cut testing wait time from hours, if not days, to as little as five minutes for positive results and 13 minutes for negative results.