Search Posts
Recent Posts
- Rhode Island Weather for June 4, 2025 – Jack Donnelly June 4, 2025
- Sour Grapes time! – Tim Jones (meet Tim at AnimeCon) June 4, 2025
- Lawsuit filed to stop Empire Wind Project by 4 environmental groups and fishermen June 4, 2025
- It is what it is: 6.4.25 – Jen Brien June 4, 2025
- New ALS treatment by PathMaker Neurosystems. Co. funded by RI Life Sciences Hub to come to RI. June 3, 2025
Categories
Subscribe!
Thanks for subscribing! Please check your email for further instructions.
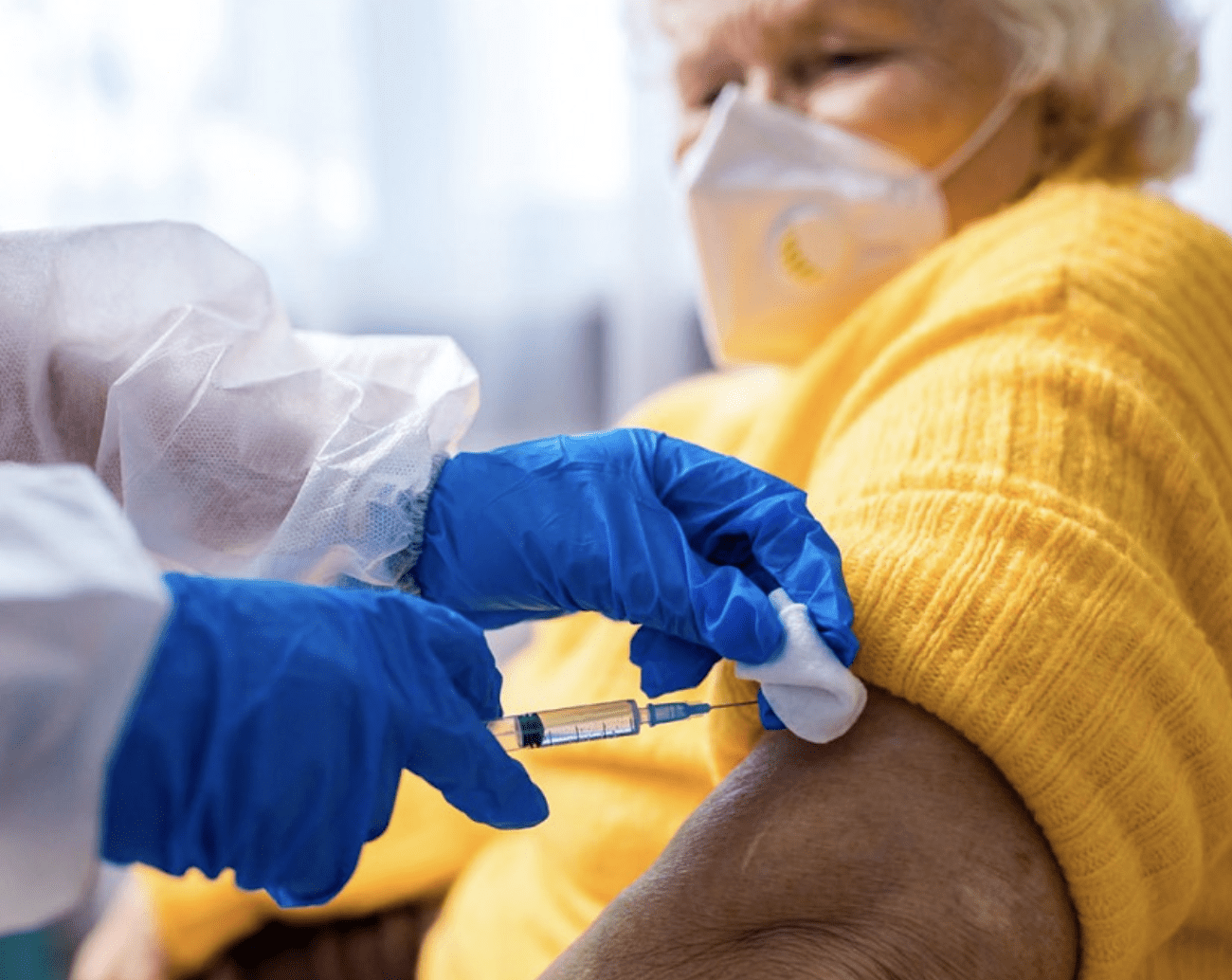
Boosters begin at nursing homes. Brown Univ. gets $4.9M from CDC to study how immunity fades with age.
Photo: Brown University
Boosters begin at RI nursing homes. Brown University research showed the CDC how immunity fades with age, over time.
It’s been approximately 8 or 9 months since most nursing home/long term care/congregate care residents received their second vaccination. Most having received their first and second shots from outside pharmacy/medical partners – a system familiar to facilities for delivering flu shots.
The CDC education efforts are going full blast on boosters for selected individuals. Early booster plans called for people to receive the 3rd shots after 8 or 9 months, but that was changed to 7 and then 6 – partly due to research presented to the CDC by the Brown University research team.
Now, and as of September 30th, the booster shots are identified for:
COVID-19 Vaccine booster shots are available for the following Pfizer-BioNTech vaccine recipients who completed their initial series at least 6 months ago and are:
- 65 years and older
- Age 18+ who live in long-term care settings
- Age 18+ who have underlying medical conditions
- Age 18+ who work in high-risk settings
- Age 18+ who live in high-risk settings
High-risk settings include congregate/long term care settings. The CDC notes: Long-term care setting residents aged 18 years and older – Residents aged 18 years and older of long-term care settings should get a booster shot of Pfizer-BioNTech vaccine. Because residents in live closely together in group settings and are often older adults with underlying medical conditions, they are at increased risk of infection and severe illness from COVID-19.
The CDC also identifies “healthcare workers” in the booster shot target group now.
Nursing homes have been busy administering annual flu shots over the last several weeks.
Booster shots are being started this month, with some nursing homes already underway, others planning to begin next week. While we aren’t hearing the general hoopla that we did with the first and second vaccines, a quick check of several nursing homes indicated that most are beginning their shots in a variety of ways.
Some nursing homes are utilizing outside “medical vendors” while others are ordering their own supplies and getting approval from the RI Department of Health to start vaccinations to be done by their own clinical staff.
RI Health Care Association
When reached for comment, John E. Gage, President & CEO of the Rhode Island Health Care Association said, “Representatives from RIDOH met with Omnicare/CVS as well as Pharmerica/Walgreens on Friday, September 24, 2021 – one (1) day after the FDA’s approval for booster shots for individuals over 65 years of age and those who live in congregate care facilities. Facilities have been contacted by their respective Long Term Care Pharmacy and have set up booster vaccines for their residents who qualify – having been fully vaccinated more than six (6) months ago. Booster clinics are set for throughout the month of October. Prior to the recent FDA approval, booster availability was limited to those individual residents who qualified as moderately to severely immunocompromised based on their individual medical history.”
RI Department of Health
Joseph Wendelken, Public Information Officer for RIDOH sent this statement earlier in the week: “We provided a guidance document to nursing homes and assisted living facilities with many options. Those included the option for facilities to enroll in our COVID vaccination program and vaccinate residents themselves, to have a company like MedTech or Ambulance Alert come in and vaccinate residents, or to work with CVS and Walgreens again to get residents vaccinated. MedTech and Alert started to do some assisted living facilities today.”
CVS & Omnicare/CVS
“Omnicare is an industry-leading pharmacy services provider to long-term care facilities (LTCF). We began offering Pfizer-BioNTech COVID-19 booster shots to our LTCF clients’ eligible populations on September 27, following the CDC’s approval of and guidance on booster shots. We are also cooperating with the CDC in their outreach efforts to LTCFs that need support for booster shots, and [we] will work closely with facilities for the best solution for each of their unique needs. Due to the demand for booster shots in our retail pharmacies, the availability of on-site vaccination clinics at facilities is being determined by local resources.” – Matt Blanchette | Manager, Retail Communications, CVS Pharmacy
_____
Brown University Research at the CDC table in booster recommendations for nursing home residents/staff. With $4.9M from the CDC, Brown researchers doing 2-year study on COVID vaccine effectiveness in seniors.
To address the pressing issue of diminishing immunity among older adults to COVID-19 as well to vaccines designed to protect against the virus, a $4.9 million award from the U.S. Centers for Disease Control and Prevention will fund a two-year project led by Brown University researchers and conducted by a team spanning multiple institutions.
The researchers will examine the duration of protective immunity in the context of emerging strains of COVID-19, releasing interim data to the CDC as it becomes available to inform policy decisions in real time.
“Given rising case counts of the Delta variant, we need to know as soon as possible who needs a vaccine booster shot and when they need it,” said Stefan Gravenstein, co-lead investigator on the project and a professor of geriatric medicine at Brown. “This information on how specific immunity to SARS-CoV-2 infection declines with aging, disease and in long-term care residents is critically important for developing a booster strategy based on real-time data in this population.”
The CDC awarded the contract to Gravenstein; Elizabeth White, a Brown assistant professor of health services, policy, and practice; and David Canaday at Case Western Reserve University. The project is based at Brown’s Center for Long-Term Care Quality and Innovation, which focuses on research to improve care and quality of life for older adults living in nursing homes.
The new award builds on previous research about protective immunity over time conducted by Gravenstein and Canady. Their most recent study, published online in late August, found that COVID-19 antibodies produced by the Pfizer vaccine decreased sharply in nursing home residents and health care workers six months after receiving their second shots.
For that study, the research team studied blood samples of 120 nursing home residents and 92 health care workers. In particular, they looked at humoral immunity — also called antibody-mediated immunity — to measure the body’s defenses against the coronavirus. The researchers, including a lab team at Harvard University, found that individuals’ antibody levels decreased more than 80% after six months; the decline was similar in residents (median age 76) and health care workers (median age 48), according to the study. However, absolute antibody levels were much lower in the nursing home residents who had not also had a prior infection than the comparison groups.
After presenting their unpublished results directly to senior staff at the CDC, the researchers were urged to get the data out in the public domain as soon as possible to inform the decision-making process for booster vaccine recommendations. As a result, the researchers published the findings as a preliminary report ahead of peer review on medRxiv, an online preprint server for health sciences studies co-founded by Cold Spring Harbor Laboratory, Yale University and the BMJ, a global healthcare knowledge provider. The study is currently under review at a traditional peer-reviewed journal.
Another study by Gravenstein, Canaday and colleagues published last May found that within two weeks of receiving the second dose of vaccine and being considered “fully vaccinated,” seniors who had not previously contracted the virus that causes COVID-19 mounted a substantially lower response to vaccine than experienced by younger health care workers. By six months after vaccination, 70% of these nursing home residents had blood test results showing a poor ability to neutralize the virus.
The preprint research is useful as the team continues to examine immunity in elderly patients.
“Aside from the obvious value of better understanding who and when to boost against SARS-CoV-2 infection, we’ve also substantially innovated in how to efficiently recruit and immunologically follow a long-term care population,” Gravenstein said. “This methodological advancement leverages Brown’s strengths in the intersection between biology, aging and public health research.”
Results up to this point support the CDC’s recommendation for booster shots — especially for the elderly — due to fading immunity, Gravenstein said. The team will now study 800 to 1,200 nursing home residents who have received one of the SARS-CoV-2 vaccines or who will newly receive a vaccine or booster and will assess their overall health and immune responses to see whether and how immunity to COVID changes over time. The team will continue to produce and share data in six-month segments.
As older residents in the study become re-vaccinated, Gravenstein said, this work will show whether that vaccination bolsters not just antibody levels and immediate protection, but also fortifies more durable defenses against new, incoming strains.
COVID-19 isn’t going away, Gravenstein said — if anything, it’s becoming more like the flu in its persistency, and as the virus becomes more endemic to modern society, “this information on immunity will be critical to know.”
Read the preliminary study results, here: https://www.medrxiv.org/content/10.1101/2021.08.15.21262067v3.full.pdf
