Search Posts
Recent Posts
- GriefSpeak: Waiting for Dad – Mari Nardolillo Dias June 13, 2025
- Rhode Island Weather for June 13, 2025 – Jack Donnelly June 13, 2025
- Urgent call by Rhode Island Blood Center for Type O- and B- blood June 13, 2025
- Real Estate in RI: Ready, Set, Own. Pathway to Homeownership workshop June 13, 2025
- Outdoors in RI – Risky cattails, Fight the Bite mosquito report, 2A gun ban bill update June 13, 2025
Categories
Subscribe!
Thanks for subscribing! Please check your email for further instructions.
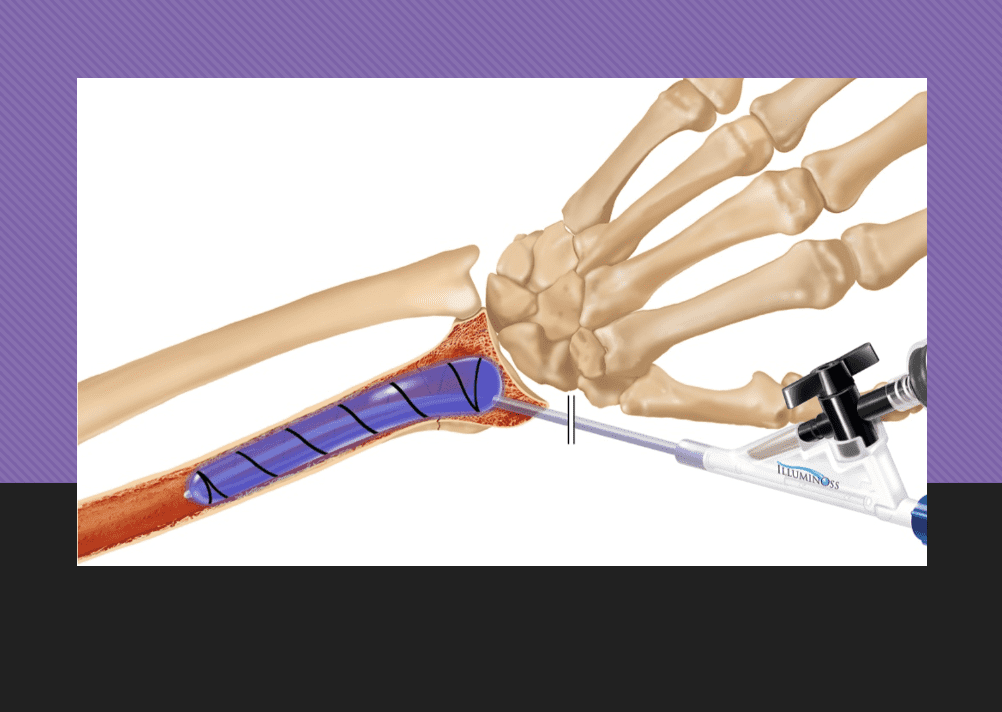
RI-based IlluminOss’ minimally invasive fracture repair, with return to activity within days
IlluminOss Medical, a medical device company focused on minimally invasive orthopedic fracture repair, is addressing an unmet need in the burgeoning senior population.
“The latest stats from the National Osteoporosis Foundation are that as many as 2 million Medicare recipients sustained roughly 2.3 million fractures related to osteoporosis in 2015,” said Anthony M. Deluise Jr. MD FAAOS, CAQSH. “In the case of a humerus fracture, many physicians previously would have said the best course of action is non-surgical, which could a sling and a year of immobility. If the patient is healthy enough for surgery, they would undergo an arduous procedure, with a long time under anesthesia and morphine during recovery with potential negative effects on memory. IlluminOss offers a minimally invasive approach for fracture repair and stabilization, with a return to normal activity within days.”
Fragility fractures result in an enormous burden on the medical system in terms of hospitalization, subsequent rehabilitation, and potential complications.
“The intangible costs to seniors’ quality of life, mental health, and loss of independence are incalculable, but steep,” Dr. Deluise added. “I have used the IlluminOss system on many patients, and the results have been astounding.”
The IlluminOss System utilizes a light-curable liquid monomer, contained within an expandable balloon, to create a patient-conforming, rigid implant within the bone canal. Surgeons report that their patients regain mobility faster and are able to resume their daily living activities far faster than conventional implants.
The IlluminOss technology has been in clinical use in Europe since 2010, and in the U.S. since 2018, with over 8,000 procedures to date. In the U.S., the IlluminOss System is now indicated for use in skeletally mature patients in the treatment of traumatic, fragility, pathological, and impending pathological fractures of the humerus, radius, ulna, clavicle, pelvis, fibula, metacarpals, metatarsals, and phalanges. The IlluminOss Photodynamic Bone Stabilization System can also be used in conjunction with FDA-cleared fracture fixation systems to provide supplemental fixation in these anatomic sites. The IlluminOss System may be used in the femur and tibia to provide supplemental fixation to an anatomically appropriate FDA-cleared fracture fixation system.
About IlluminOss Medical, Inc.
Headquartered in East Providence, RI, IlluminOss is a privately held, commercial-stage medical device company offering a unique, minimally invasive technology for fracture repair and stabilization. The company utilizes a light-curable monomer contained within an expandable balloon to create a patient-conforming intramedullary implant for bone stabilization. The revolutionary, minimally invasive technology is particularly applicable for repair and treatment of osteoporotic and compromised bone. The IlluminOss system is CE-marked and FDA-cleared for a variety of anatomical sites, with further indications pending. For additional information, including a complete list of indications, contraindications, warnings, precautions and risks, visit www.illuminoss.com.
Video Link:
For more detailed procedural information including Indications, Warnings, Cautions, Risks & Contraindications, visit www.illuminoss.com.

Very interesting article, especially for us with elderly parents.