Search Posts
Recent Posts
- Writer Herb Weiss’ 45 years of Advocacy on Aging now Archived at Rhode Island College Library Special Collection June 23, 2025
- Providence Biopharma, Ocean Biomedical, Notified of Termination of License Agreements with Brown University, RI Hospital June 23, 2025
- Networking Pick of the Week: Early Birds at the East Bay Chamber, Warren, RI June 23, 2025
- Business Monday: Dealing with Black and White Thinking – Mary T. O’Sullivan June 23, 2025
- Rhode Island Weather for June 23, 2025 – Jack Donnelly June 23, 2025
Categories
Subscribe!
Thanks for subscribing! Please check your email for further instructions.

Hair product recall due to benzene, a known carcinogen
The Procter & Gamble Company (NYSE: PG) today issued a voluntary product recall to the consumer level of aerosol dry conditioner spray products and aerosol dry shampoo spray products from Pantene, Aussie, Herbal Essences, and Waterless produced in the United States, in addition to previously discontinued aerosol dry shampoo products from Old Spice and Hair Food, due to the presence of benzene detected in some products.
Risk Statement: Benzene is classified as a human carcinogen. Exposure to benzene can occur by inhalation, orally, and through the skin and it can result in cancers including leukemia and blood cancer of the bone marrow and blood disorders which can be life-threatening. Based on exposure modeling and the cancer risk assessments published by the Environmental Protection Agency (EPA) (IRIS database), daily exposure to benzene in the recalled products at the levels detected in our testing would not be expected to cause adverse health consequences. Benzene is ubiquitous in the environment. Humans around the world have daily exposures to it indoors and outdoors from multiple sources. To date, The Procter & Gamble Company has not received any reports of adverse events related to this recall and is conducting this recall out of an abundance of caution.
Detailed instructions for how to request a reimbursement for eligible products can be found below. The affected products are packaged in aerosol cans. See table below for images, product names, UPC and production code ranges. Refer to the image attached for guidance on where to find the production code details on the bottom of the can. The first four numbers of the production code are the only ones necessary to determine if your product is impacted and falls within the ranges outlined.
The aerosol dry conditioner spray products impacted are:
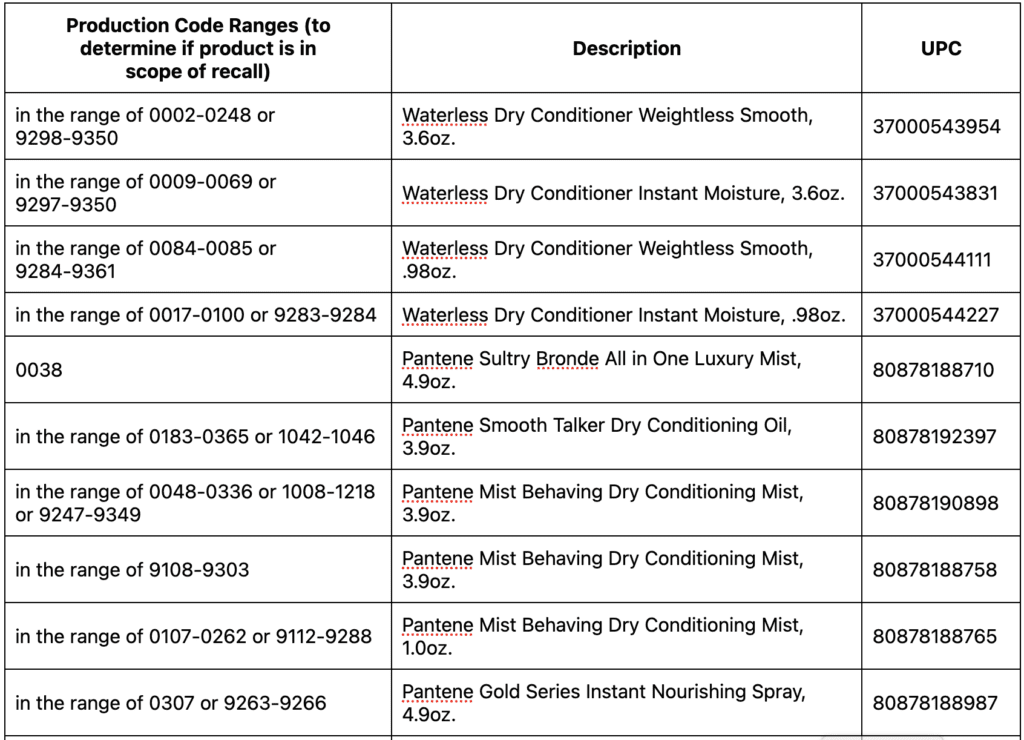
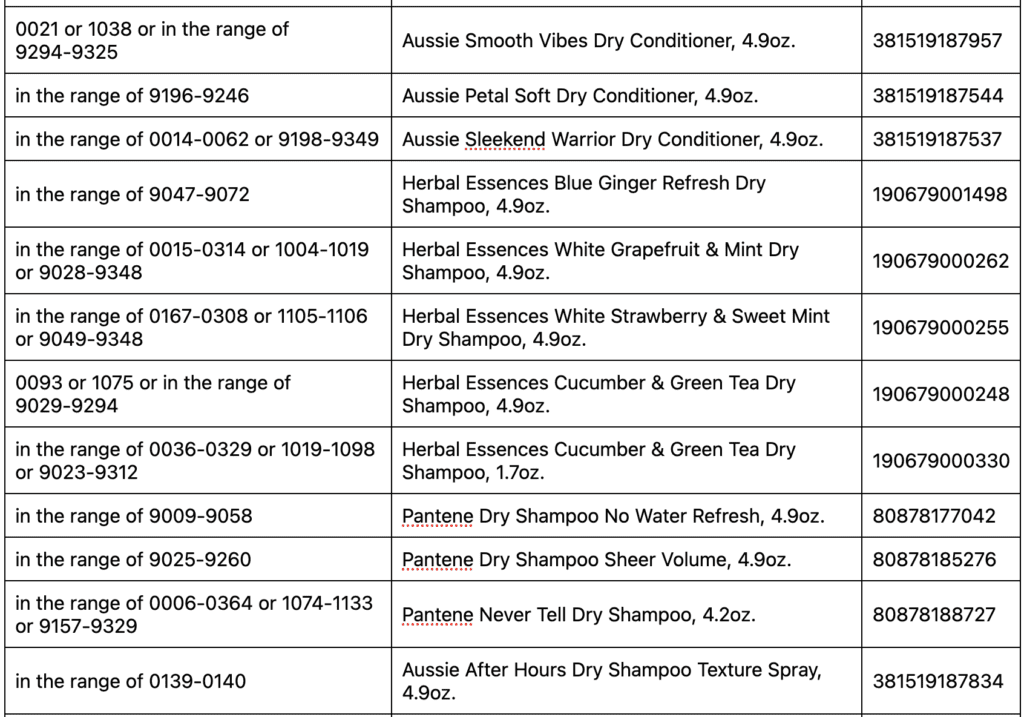
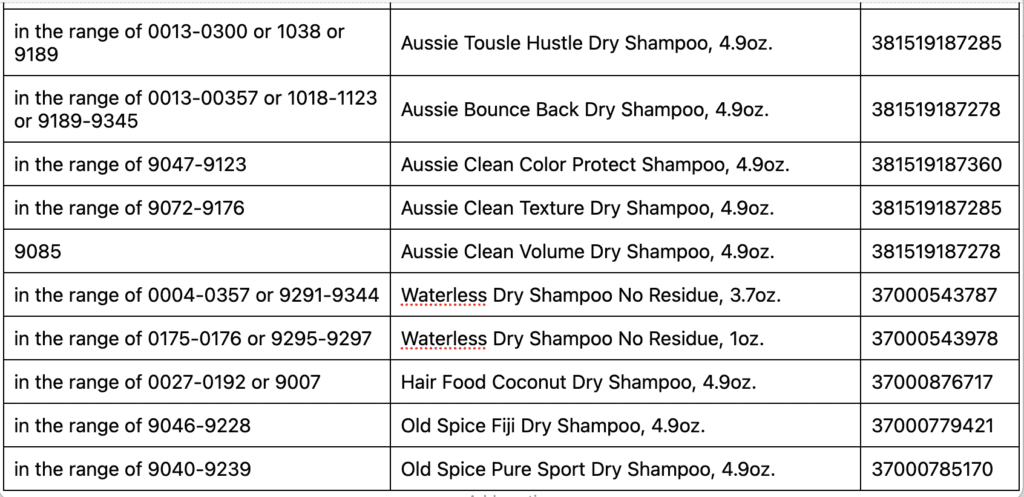
Following recent reports that indicated traces of benzene in some aerosol spray products, we began a review of our total portfolio of aerosol products. While benzene is not an ingredient in any of our products, our review showed that unexpected levels of benzene came from the propellant that sprays the product out of the can. We detected benzene in aerosol dry shampoo spray products and aerosol dry conditioner spray products. Nothing is more important to us than the safety of the consumers who use our products and the quality of the products we ship.
No other products from Pantene, Aussie, Herbal Essences, Hair Food, and Waterless are in the scope of this recall and such other products may continue to be used as intended, including those aerosol dry shampoo spray products with production code ranges different from those specifically communicated. The vast majority of our products are not part of this recall, including mousses, hairsprays, liquid shampoos, liquid conditioners, styling products, treatments, and unaffected aerosol dry shampoo sprays.
The recalled products were distributed nationwide in the United States through retail outlets and online. Retailers have been alerted to remove recalled products from shelves. Our brands will also offer reimbursement for consumers who have purchased products impacted by this recall. Consumers should stop using and appropriately discard the affected aerosol dry conditioner spray products and aerosol dry shampoo spray products.
If consumers have further questions, they can also seek more information via the Consumer Care team at 1-888-674-3631 from Monday – Friday from 9:00am – 6:00pm EST.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
.This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
