Search Posts
Recent Posts
- Real Estate in RI: Seaside waterfront communities are all the rage. Who’s buying – Emilio DiSpirito June 6, 2025
- Outdoors in RI: 2A votes, Charter Yachts, active summer programs, garden tours, aquatic weeds… June 6, 2025
- All About Home Care, with two Rhode Island locations, closing after 22 years in business June 6, 2025
- GriefSPEAK: Angel wings with footprints – Mari Nardolillo Dias June 6, 2025
- Rhode Island Weather for June 6, 2025 – Jack Donnelly June 6, 2025
Categories
Subscribe!
Thanks for subscribing! Please check your email for further instructions.
FDA approves first gene therapies for Sickle Cell Disease
SCDAA (Sickle Cell Disease Association of America) Statement About Gene Therapy Approval
On Dec. 8, 2023, the Food and Drug Administration (FDA) approved two cell-based gene therapies for sickle cell disease (SCD), Casgevy from CRISPR/Vertex and Lyfgenia from bluebird bio. These are the first treatments of their kind available to individuals with SCD in the United States. SCDAA welcomes the approval of these potentially curative therapies which mark major advances in the treatment of sickle cell disease; however, there are valid concerns about accessibility and the potential for adverse effects.
Dr. Lewis Hsu, chief medical officer of the Sickle Cell Disease Association of America Inc. said:
We at the Sickle Cell Disease Association of America Inc. celebrate that two gene therapies are approved for sickle cell disease by the FDA today, Dec. 8. This double milestone was a long time coming, and sickle cell disease now joins the ranks of other genetic diseases with gene therapy treatments. Sickle cell disease was called “the first molecular disease” about 70 years ago, and a gene therapy treatment was predicted for sickle cell disease in the 1950s when DNA was first described.
These two gene therapies mark a big step forward for sickle cell disease research and treatment. The results of the clinical trials are very impressive. The patients selected had a lot of pain (two or more vaso-occlusive crisis pain hospitalizations per year for two years). The data show that, after gene therapy was administered, nearly all patients were free of hospitalizations for vaso-occlusive crises for at least nine consecutive months. The patients’ health-related quality of life also improved in every way: physically, emotionally, socially and functionally with both gene therapies.
Regina Hartfield, president and CEO of the Sickle Cell Disease Association of America Inc. said:
Gene therapy is an exciting and potentially curative addition to the treatments available to sickle
cell warriors. This is a historic milestone, but everyone may not be eligible for gene therapy.
We must continue to move forward with research to ensure that there is a solution for every member of our community.
Is gene therapy a cure for sickle cell disease?
The Sickle Cell Disease Association of America Inc. recognizes gene therapy as a “potentially curative” therapy. The treatment is so new that more data is needed to understand its impact and patient prognosis. Additionally, the word “cure” suggests a simple solution that does not reflect the reality of these therapies. Even after completing treatment, the FDA recommends 15 years of patient monitoring for health issues.
What is the treatment like?
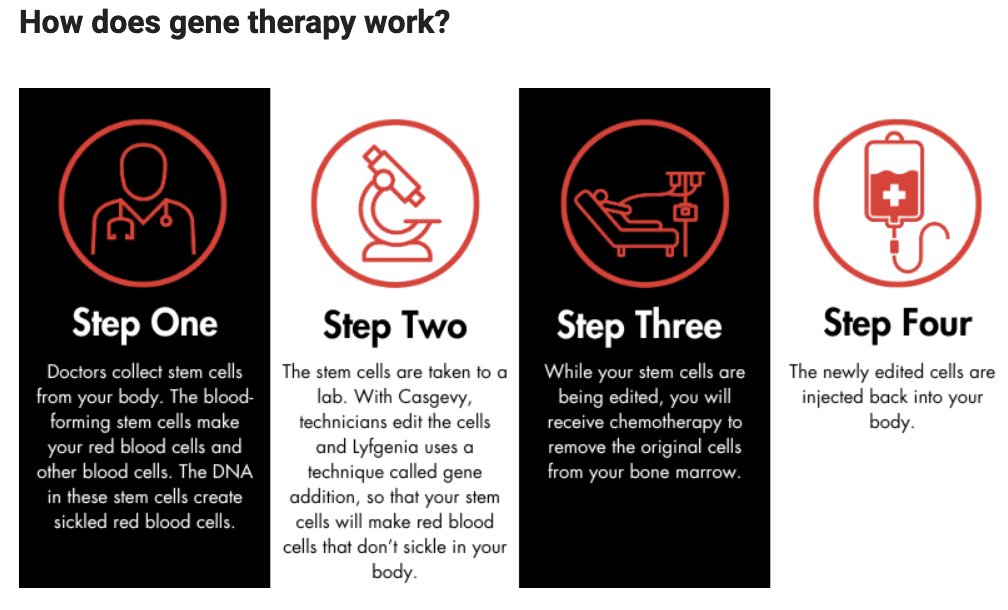
The patient journey will be similar for both Casgevy and Lyfgenia. Gene therapy is administered during a one-time infusion; however, there are steps that patients must take to prepare for the treatment. First, the patient’s care team will collect stem cells, which are the progenitors of red blood cells, from their body. Then, those cells will be treated in a lab. The patient will undergo chemotherapy to remove the original, abnormal stem cells from the bone marrow. After this process is complete, the treated stem cells are injected back into the patient through an intravenous process like a transfusion (not surgery). The whole procedure takes about a year. It is similar to autologous bone marrow transplantation, as there is no need to find a donor of stem cells.
What is the difference between the two therapies?
The two gene therapy strategies are scientifically different. Casgevy is gene editing, the first of its kind, and Lyfgenia uses gene addition. Both gene therapy strategies have about the same patient journey and potential issues: access, cost, infertility, unknown possibility of organ damage and unknown long-term effects.
How effective is it?
Gene therapy provides a significant reduction in acute episodes of sickle cell pain within a few years of administration. More years of follow-up will be needed to determine whether it will also reduce the organ damage of sickle cell disease and if the stem cells treated with continue to produce non-sickling red blood cells for the rest of the person’s life, or if the stem cells die off over a certain number of years. Currently the treatment requires chemotherapy, which means there are also concerns about chemotherapy-associated complications, such as infertility or secondary cancer.
When will gene therapy be available for use?
Gene therapy will likely be available in early 2024.
Who is eligible?
We are awaiting the details but probably for use in individuals ages 12 and up.
Where is gene therapy administered?
Individuals with SCD can receive gene therapy at existing bone marrow treatment facilities with sickle cell expertise, which may pose accessibility issues to patients. SCDAA encourages gene therapy centers to partner with sickle cell centers, such as in the National Alliance of Sickle Centers, so that there is expertise to monitor for sickle cell organ damage.
How much will it cost? Will insurance cover gene therapy?
Gene therapy treatments are produced through expensive, highly technical processes. The cost estimates for treatment are $2 million and up. However, the high price should be worthwhile as the savings in lifelong care may exceed the one-time cost of gene therapy. FDA-approved high-cost medications come with insurance barriers and rules that are not evidence-based.
What do these approvals mean for people living with sickle cell?
These approvals are expected to be life-changing for many and usher in a new age of treatment for sickle cell disease. Until now, the only way to cure sickle cell disease was through a bone marrow transplant, which is not a widely accessible option because it requires a matched bone marrow donor. Gene therapy does not require a donor; therefore, it has the potential to be a more widely available treatment.
What does it mean for sickle cell treatment in the future?
Casgevy and Lyfgenia are the first gene therapy treatments approved by the FDA for sickle cell disease. They open the door for other gene therapies to gain approval and help advance research into other potentially curative treatments. At the same time, there are concerns that these approvals will create an increase in competition for health care resources that could make it difficult to access other forms of treatment outside of gene therapy. Many people will not be eligible to receive gene therapy. To provide the highest quality of care to these individuals, we need to continue research into a variety of treatment options beyond gene therapy.
What are the implications for the medical community at large?
More broadly, Casgevy is the first FDA-approved CRISPR gene editing therapy for a genetic disease. This could have wide-reaching impacts for individuals with other conditions like cystic fibrosis, Tay-Sachs disease and others.
___
The FDA Statement: FDA approves first gene therapies to treat patients with Sickle Cell Disease
The U.S. Food and Drug Administration approved two milestone treatments, Casgevy and Lyfgenia, representing the first cell-based gene therapies for the treatment of sickle cell disease (SCD) in patients 12 years and older. Additionally, one of these therapies, Casgevy, is the first FDA-approved treatment to utilize a type of novel genome editing technology, signaling an innovative advancement in the field of gene therapy.
Sickle cell disease is a group of inherited blood disorders affecting approximately 100,000 people in the U.S. It is most common in African Americans and, while less prevalent, also affects Hispanic Americans. The primary problem in sickle cell disease is a mutation in hemoglobin, a protein found in red blood cells that delivers oxygen to the body’s tissues. This mutation causes red blood cells to develop a crescent or “sickle” shape. These sickled red blood cells restrict the flow in blood vessels and limit oxygen delivery to the body’s tissues, leading to severe pain and organ damage called vaso-occlusive events (VOEs) or vaso-occlusive crises (VOCs). The recurrence of these events or crises can lead to life-threatening disabilities and/or early death.
“Sickle cell disease is a rare, debilitating and life-threatening blood disorder with significant unmet need, and we are excited to advance the field especially for individuals whose lives have been severely disrupted by the disease by approving two cell-based gene therapies today,” said Nicole Verdun, M.D., director of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research. “Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited.”
Casgevy, a cell-based gene therapy, is approved for the treatment of sickle cell disease in patients 12 years of age and older with recurrent vaso-occlusive crises. Casgevy is the first FDA-approved therapy utilizing CRISPR/Cas9, a type of genome editing technology. Patients’ hematopoietic (blood) stem cells are modified by genome editing using CRISPR/Cas9 technology.
CRISPR/Cas9 can be directed to cut DNA in targeted areas, enabling the ability to accurately edit (remove, add, or replace) DNA where it was cut. The modified blood stem cells are transplanted back into the patient where they engraft (attach and multiply) within the bone marrow and increase the production of fetal hemoglobin (HbF), a type of hemoglobin that facilitates oxygen delivery. In patients with sickle cell disease, increased levels of HbF prevent the sickling of red blood cells.
Lyfgenia is a cell-based gene therapy. Lyfgenia uses a lentiviral vector (gene delivery vehicle) for genetic modification and is approved for the treatment of patients 12 years of age and older with sickle cell disease and a history of vaso-occlusive events. With Lyfgenia, the patient’s blood stem cells are genetically modified to produce HbAT87Q, a gene-therapy derived hemoglobin that functions similarly to hemoglobin A, which is the normal adult hemoglobin produced in persons not affected by sickle cell disease. Red blood cells containing HbAT87Q have a lower risk of sickling and occluding blood flow. These modified stem cells are then delivered to the patient.
The Treatment Process
Both products are made from the patients’ own blood stem cells, which are modified, and are given back as a one-time, single-dose infusion as part of a hematopoietic (blood) stem cell transplant. Prior to treatment, a patients’ own stem cells are collected, and then the patient must undergo myeloablative conditioning (high-dose chemotherapy), a process that removes cells from the bone marrow so they can be replaced with the modified cells in Casgevy and Lyfgenia. Patients who received Casgevy or Lyfgenia will be followed in a long-term study to evaluate each product’s safety and effectiveness.
A few patients who were in the trial have spoken out to say the treatment is painful, but the results, for them, were life changing and worth it.
“These approvals represent an important medical advance with the use of innovative cell-based gene therapies to target potentially devastating diseases and improve public health,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “Today’s actions follow rigorous evaluations of the scientific and clinical data needed to support approval, reflecting the FDA’s commitment to facilitating development of safe and effective treatments for conditions with severe impacts on human health.”
Data Supporting Casgevy
The safety and effectiveness of Casgevy were evaluated in an ongoing single-arm, multi-center trial in adult and adolescent patients with SCD. Patients had a history of at least two protocol-defined severe VOCs during each of the two years prior to screening. The primary efficacy outcome was freedom from severe VOC episodes for at least 12 consecutive months during the 24-month follow-up period. A total of 44 patients were treated with Casgevy. Of the 31 patients with sufficient follow-up time to be evaluable, 29 (93.5%) achieved this outcome. All treated patients achieved successful engraftment with no patients experiencing graft failure or graft rejection.
The most common side effects were low levels of platelets and white blood cells, mouth sores, nausea, musculoskeletal pain, abdominal pain, vomiting, febrile neutropenia (fever and low white blood cell count), headache and itching.
Data Supporting Lyfgenia
The safety and effectiveness of Lyfgenia is based on the analysis of data from a single-arm, 24-month multicenter study in patients with sickle cell disease and history of VOEs between the ages of 12- and 50- years old. Effectiveness was evaluated based on complete resolution of VOEs (VOE-CR) between 6 and 18 months after infusion with Lyfgenia. Twenty-eight (88%) of 32 patients achieved VOE-CR during this time period.
The most common side effects included stomatitis (mouth sores of the lips, mouth, and throat), low levels of platelets, white blood cells, and red blood cells, and febrile neutropenia (fever and low white blood cell count), consistent with chemotherapy and underlying disease.
Hematologic malignancy (blood cancer) has occurred in patients treated with Lyfgenia. A black box warning is included in the label for Lyfgenia with information regarding this risk. Patients receiving this product should have lifelong monitoring for these malignancies.
Both the Casgevy and Lyfgenia applications received Priority Review, Orphan Drug, Fast Track and Regenerative Medicine Advanced Therapy designations.
The FDA granted approval of Casgevy to Vertex Pharmaceuticals Inc. and approval of Lyfgenia to Bluebird Bio Inc.
